Non-bonded interactions¶
Inter-molecular or non-bonded interactions consist of energy components that interact between pairs of atoms and depend only on interatomic distances.

Non-bonded interactions consist of several energy components as follows:
- Electrostatic (Coulombic) interactions. These arise due to charges on atoms and they are considered long-range in nature.
- Dispersive (van der Waals) interactions. Such interactions are due to electron cloud fluctuations, forming instantaneous dipoles. They can be found even in inert gas systems and neutral atoms.
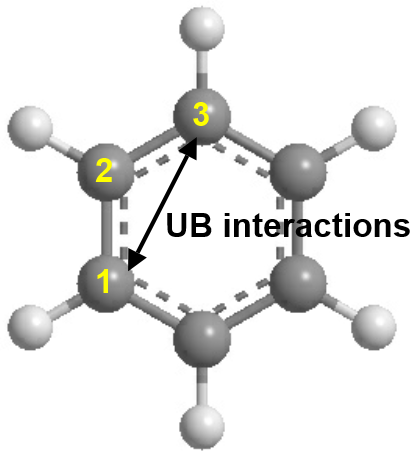
- Urey-Bradley (UB) 1-3 atom pair interactions. These are specific to CHARMM FF schemes and correlate with angle interactions. The diagram below shows UB interactions for a benzene molecule.
- Others. These can include dipole-dipole interactions such as the hydrogen bonds, charge-dipole interactions, etc. However, most FF schemes do not explicitly include such interactions as they are implicitly included in the above mentioned interactions.
Note
For covalent molecules, non-bonded interactions do not apply to 1-2 and 1-3 bonded atoms (except for any UB interactions). For 1-4 bonded atoms (e.g. those involved in dihedrals), a constant scaling factor is usually applied to non-bonded interactions between them: this will depend on the FF scheme.