Improper (dihedral) functions¶
Previously, we saw that dihedral functions are defined by four atoms connected successively (1-2-3-4), forming a chain. However, an improper dihedral term is a special form of dihedral where four atoms are specified such that three atoms are connected to a fourth central atom, as shown below.
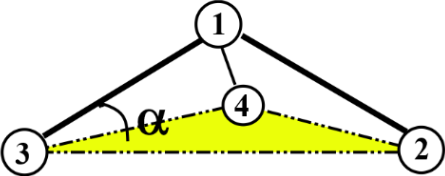
where \(\alpha\) is the improper angle of the bond vector 1-3 relative to the plane consisting of atoms 2, 3 and 4.
Improper functions are included to restrict the geometry of certain parts of molecules that otherwise would not be obtainable using simple classical bonds. For example, these can be used to preserve the planar (flat) conformation of carbonyl groups in a molecule.
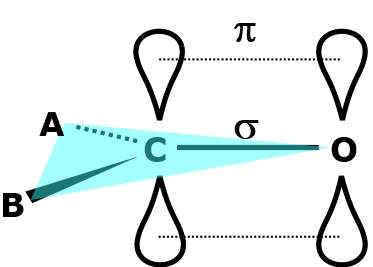
Here, the carbon atom is sp\(^2\) hybridised, where the \(\pi\)-bonding on the carbonyl C=O bond restricts the rotation of atom A and atom B relative to the carbonyl group. Subsequently, carbonyl groups adopt a planar conformation with \(\alpha = 0^{\circ}\).